CHARMM: Trajectory and Analysis Tutorial
Objective and Overview
The objective of this tutorial is to introduce common
analyses of structures and simulation trajectories using various
CHARMM analysis facilities. All examples shown in this tutorial are
based on analysis of a short MD trajectory of the protein G B1
domain solvated in a cubic box of TIP3P water (shown on the right).
|
Solvated protein G B1 domain (PDB:3GB1)
|
Tutorial Components:
- Extract RMSD vs Initial Structure and Radius of Gyration of the Protein
- Extract Histograms of φ/ψ Distributions
- Analyze the Secondary Structure Using the Kabsch and Sander DSSP Algorithm
- Find Hydrogen Bonds in the Initial Structure, and from the Trajectory
- Analyze Water Diffusion and Water Structure Around the Protein
- Reorient/Recenter the Trajectory, and also Remove All Solvent
- Extract NMR Relxation Rates and Order Parameters for the Backbone Amides
- Analyze the Time-dependent Fluorescence Anisotropy Decay of a Trp Residue
- Cluster Structures from the Trajectory, Based on Selected φ and ψ dihedrals
These examples represent somewhat routine analyses but
can be combined with the CHARMM scripting
language to achieve many other more extensive analyses. Along with performing
the examples, please examine the structure and trajectory
using VMD or other visualization software. Also, one can choose favorite software for making plots
from the data files produced following CHARMM analysis, although all data plots shown here are
were generated by the Gnuplot, and these scripts provided.
Please also keep in mind that the provided 100 ps trajectory with 100 coordinate
frames sampled every 1 ps is far too short for many of the phenomena are analyzed here.
To run any of the CHARMM analysis scripts:
charmm < scriptname.inp > scriptname.log
To run the gnuplot scripts:
gnuplot scriptname.gnu
1. Root Mean Square Deviation and Radius of Gyration
rmsd-singlecrd.inp
rmsd-rgyr-traj-corman.inp
rmsd-rgyr-traj-correl.inp
Two simple characteristics of a structure are
-
The Root Mean Squared Difference (RMSD) with respect to a reference structure (e.g., the
starting point of a simulation), tells us something about the
dissimilarity between the structures - 1 Å RMSD is barely visible to
the eye, while an RMSD 10 Å is leading to a significantly different structure.
-
The Radius of Gyration (RGYR) is a combined measure of its overall size and shape. A sphere
has the smallest RGYR of any structure with the same volume. Increasing
the volume, making the shape more anisotropic, or having more of its
mass at the periphery, all lead to an increased RGYR. The
proper definition of RGYR in mechanics is mass-weighted, which
can be obtained by adding keyword MASS to the COOR RGYR command.
Three scripts are provided to demonstrate how to evaluate RMSD
and RGYR using CHARMM. The rmsd-singlecrd.inp script is extremely
simple and calculates only the Cα (CHARMM atom type CA) RMSD between two structures.
The rmsd-rgyr-traj-corman.inp and rmsd-rgyr-traj-correl.inp scripts
calculate RMSD and RGYR of a trajectory from a reference structure
using two methods (a loop with CORMAN versus no loop with CORREL). In CORREL, the
results could have been output to one file for each property, but
the ability to edit the dimensions of the extracted time series
in CORREL (edit ... veccod) is used to put all the information into one file.
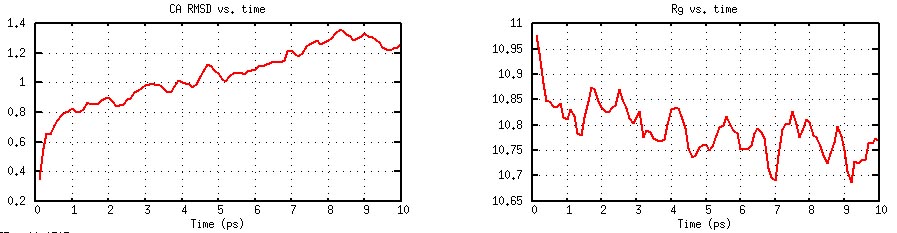
CA RMSD and Rg as Functions of Time (Generated by plot_rmsd.gnu).
Back to Top
2. φ/ψ Distributions
phi-psi-dist.inp
Peptide backbone dihdrals φ and ψ determine the overall fold of
a protein. In this example, histograms of φ and ψ are extracted, and a
distribution from the trajectory for any selected residue using
correl.doc is obtained. This script has the ability to take commandline
arguments. The argument, @rid (residue ID), has a
default value of 41 by the virtue of the following command in phi-psi-dist.inp:
if @?rid eq 0 set rid = 41
However, the value of @rid can be overwritten by following CHARMM excution:
charmm rid=15 < phi-psi-dist.inp | tee phipsi.log
After the TRAJ command in the CORREL module has been executed
averages and fluctuations are printed out for each time
series. Both histograms are output to a single file named
phi-psi-dist.dat, and the φ/ψ time series are output to
phipsi.dat. Following is a plot of of the results for Gly41.
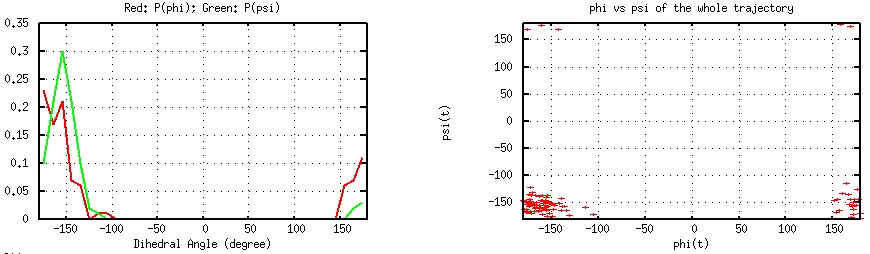
Gly41 φ/ψ Histograms (left) and Time Series (Generated by plot_phipsi.gnu).
Back to Top
3. Secondary Structure
2nd-structure.inp
COOR SECS (corman.doc) computes the secondary structure
characteristics of a protein using the DSSP algorithm (Kabsch and
Sander 1983), which is based on backbone hydrogen bond patterns. The
CHARMM implementation is a slight simplification and uses the
donor(hydrogen atom)-acceptor-atom definition of a hydrogen bond. Results are
summarized in the output file shown below:
...
CHARMM> COOR SECS SELE .not. resn tip3 end VERBOSE
SELRPN> 855 atoms have been selected out of 17088
Secondary structure (Kabsch&Sander) analysis.
Using 56 aa in a context of 56 aa.
14 aa in alpha-helix ( 25%), and 24 aa in beta-strands ( 42%).
1: MET-THR-TYR-LYS-LEU-ILE-LEU-ASN-GLY-LYS-THR-LEU-LYS-GLY-GLU
E E E E E E E E E E
16: THR-THR-THR-GLU-ALA-VAL-ASP-ALA-ALA-THR-ALA-GLU-LYS-VAL-PHE
E E E E H H H H H H H H
31: LYS-GLN-TYR-ALA-ASN-ASP-ASN-GLY-VAL-ASP-GLY-GLU-TRP-THR-TYR
H H H H H H E E E E
46: ASP-ASP-ALA-THR-LYS-THR-PHE-THR-VAL-THR-GLU-
E E E E E E
...
Results from the COOR SECS command are also returned as a numerical flag in the CHARMM WMAIN array.
An example where WMAIN is plotted together with the order parameters from NMR relaxation analysis with the plot_nmr-s2.gnu input file
is shown in NMR section below.
Back to Top
4. Hydrogen Bond Analysis
hbond.inp
Hydrogen bonding patterns often provide useful information. In this
exercise the COOR HBOND (corman.doc) command is used to find hydrogen
bonds in a single structure, as well as average number of hydrogen
bonds and their average life times from a trajectory. Hydrogen bonds
can be defined in many ways, but a simple geometric
criterion will be used: a hydrogen bond exists if the distance between the
hydrogen donor (D) and acceptor atoms (A) is less than 2.4 Å. This works well in
practice. Note that when there is information about the hydrogen
position, the hydrogen bond definition is not very sensitive to
angular criteria, as are often used when determining hydrogen bonds
in X-ray structures (lacking hydrogens).
Hydrogen
bonds within the protein, between protein and water, and also on
water-mediated hydrogen bonding contacts between different parts of
the protein will be examined. Hydrogen bonded interactions of the form A/D
- water - A/D, where A/D denotes a hydrogen bond donor or acceptor
in the protein will also be examined. COOR HBOND uses the information about acceptors and
donors in the PSF, so quite simple and general
atom-selections will be used. All hydrogen bonds between the acceptors/donors in
the two selections are calculated. A second form of the command
(COOR CONTact) applies only distance criterion to all the selected atoms.
The results are presented as hydrogen bonds per each acceptor/donor
in the first atom-selection; if the VERBose keyword is present a
break-down in terms of accptors/donors in the second atom-selection
is given. The VERBose keyword is not useful in a CHARMM
loop when it is desired to extract total number of hydrogen bonds through
CHARMM variables (subst.doc). <occupancy> is the average
number of hydrogen bonds formed by a given accptor/donor during the
trajectory. The <lifetime> (in ps) is the average
duration of each instance of a given hydrogen bond.
Back to Top
5. Solvent Dynamics and Structure
solvent.inp
All cells live an aqueous environment and their insides
(cytoplasm) are also largely aqueous. Biomacromolecules thus
have evolved to function (Membrane
proteins are, of course, different in this respect); consquently
CHARMM has well-developed facilities to analyze solvation behavior
(see also the Hydrogen Bonds exercise). Here, the COOR
ANAL (corman.doc) module is used to extract solvent dynamics (translational
and rotational diffusion) and structure, in terms of radial
distribution functions. The example analyzes these properties for
different regions of the water depending on the distance from
protein surface (<5 A, 5-10 A, >10 A). As shown in the following
plots, water molecules near the protein surface tend to diffuse
and rotate slower (with larger effective rotational correlation time
and smaller diffusion constant). The Rg of different residues also
indicate the degree of solvation.
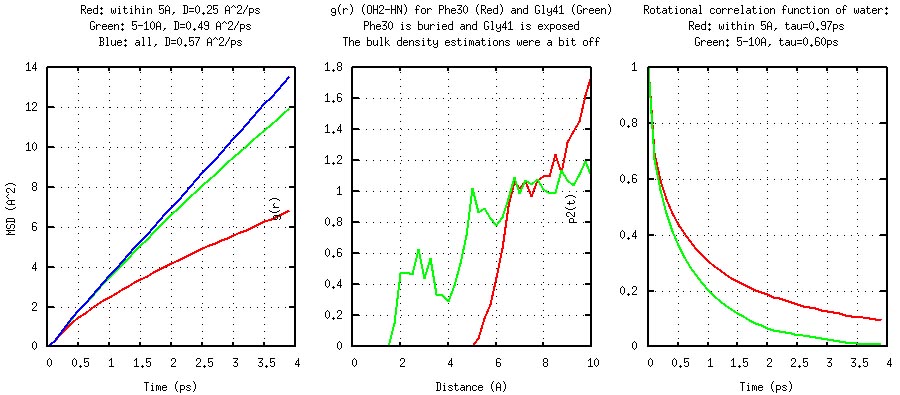
Solvent Structure and Dynamics: MSD(t) (left), g(r) (center) and P2(t) (Generated by plot_solvent.gnu).
Back to Top
6. Recentering/Orienting and Removing Water from the Trajectory
orient.inp
The raw trajectory often needs to be pre-processed to facilitate some
of the later analyses. The trajectrory was obtained from a
simulation using periodic boundary conditions (PBC), which means
that it is possible for the solute (the protein) to be partly out of
the primary simulation box. By
recentering the trajectory solvent molecules are moved according to
the PBC so that the protein is in the center of the box in each
frame. Furthermore, each frame is then re-oriented, such that the protein
is superimposed on the coordinates of the initial protein structure,
thus removing overall protein rotation/translation motions. For a
very flexible system, the removal of overall rotation may be a
non-trivial task, but G B1 is compact and quite rigid, so the
superposition is based on all atoms in the protein.
The script uses the CHARMM MERGE command (dynamc.doc) and
demonstates only the part that we re-orient the protein and remove
all water molecules. A new trajectory file is produced. The script
also contains an example on how to re-center and orient the protein
without deleting water molecules (this line is not excuted as it is
after the "stop" command). MERGE can also be used to join or
split trajectory files or to change (reduce) sampling frequency.
Back to Top
7. NMR Relaxation Rates and Generalized Order Parameters
nmr.inp
MD simulations and NMR relaxation experiments often cover similar
time-scales (ps-ns). Relaxation phenomena observed in NMR
experiments depend on motional behavior of nuclear dipoles in the
macromolecule, and the NMR module in CHARMM has been designed to
allow efficient extraction of NMR related parameters from a
trajectory (nmr.doc). This example analyzes relaxation parameters,
including relaxation rates, generalized order parameters, and configurational
entropy estimates for the backbone amide groups.
Results, with some details such as the computed correlation
functions are given in the output file nmr-rt_ct.dat, and are also
summarized in nmr-r1r2.dat. Note that the protein trajectory with
overall translation/rotation removed is used. It is assumed that the
internal and overall motions occur on very different time scales
and can confidently be separated, allowing the focus on the
internal motions. Such an assumption is necessary, as most
simulations are not long enough to sufficiently sample global
rotational diffusion. The following figure plots the general order
parameter S2 for each HN vector together with the secondary
structure (generated by 2nd-structure.inp, see above). Clearly,
even for a 100 ps simulation, it is evident that loops are more
flexible with smaller S2.

General order parameter and secondary structures (generated by plot_nmr-s2.gnu).
Back to Top
8. Fluorescence Anisotropy
trp--anisotropy.inp
The motion of chromophores may be detected by following the
time-dependence of the polarized components of fluorescence emission
following a brief pulse of polarized excitation. This time-dependent
anisotropy can also be computed from the (rank two) autocorrelation
of a unit vector along the transmission dipole, or more strictly,
the correlation between absorption and emission dipoles. Trp is the
most useful intrinsic chromophore in proteins, but Tyr also has a
certain fluorescence which is less intense than that of Trp. Extrinsic
probes, including dansyl chloride and many others, are often used. The fast
decay of this fluorescence anisotropy means that
internal motions can be decoupled from the overall rotational diffusion
of the protein (See also the NMR Exercise.). Protein G B1 only has
one buried Trp. Within 100 ps simulation, Trp43 does not change
its rotamer state, and the correlation fuction does not decay significantly.
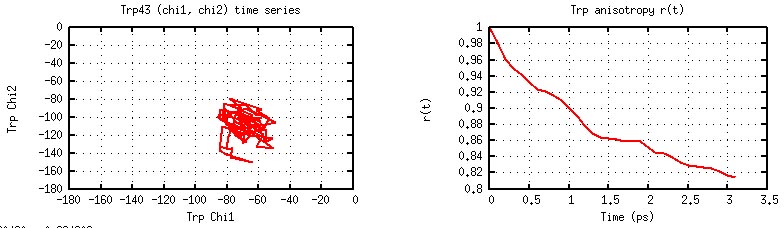
Trp43 Ch1/Ch2 Time Series and Dipole Orientational
Correlation Function (Generated by plot_trp.gnu).
Back to Top
9. φ/ψ Based Clustering
cluster.inp
As shown above in φ/ψ distribution analysis, CORREL
(correl.doc) can be used to extract time series of backbone dihedral
angles, and many other properties. Some of the
dihiedral angles that vary during the simulation can be used for clustering the
structures in the trajectory. As shown from the RMSD analysis, the
protein does not move much within 200 ps. Thus, the loop
38-41 is chosen as one that has the largest fluctuation (or smaller order parameters
from nmr analysis) to demonstrate clustering.
The number of clusters and cluster centers are summarized in clus-center.dat. Membership
and distance from center of each frame are output in clus-member.dat.
Back to Top





